Type 2 diabetic nephropathy is the leading cause of end-stage chronic kidney disease. Type 2 diabetic nephropathy affects both kidney structure [glomerular hypertrophy and sclerosis, tubulo-interstitial fibrosis , lipid deposition] and kidney function [proteinuria, reduction of glomerular filtration rate].
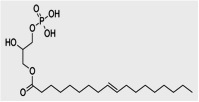
Lysophosphatidic acid (LPA) is a bioactive phospholipid produced by a lysophospholipase D (autotaxin). LPA acts through specific LPA receptors and is involved in obesity and diabetes. LPA is now recognized as a pro-fibrotic mediator in several organs including kidney and adipose tissue as recently reported by our group (Rancoule et al. Biochim Biophys Acta (Mol Cell Biol Lipids), 2014; Pradère et al. J Am Soc Nephrol. 2007; Rancoule et al. Expert Opin Investig Drugs. 2011).
Kidney produces LPA and expresses LPA receptors. LPA contributes to renal tubular-interstitial fibrosis via the activation of the LPA1 receptor and its action can be blocked in vivo by treatment with a LPA receptor antagonist. In the kidney, the production of LPA (Grove et al. J Lipid Res. 2014) and the expression of LPA receptors are increased in db/db mice, a common model of T2DN. We recently developed an LC/MS-MS method to analyse the LPA composition in urine, and found an increase of urinary LPA in patients with proteinuria.
We postulate that LPA could play an important role in the pathogenesis of type 2 diabetic nephropathy in animal and human. LPA production and/or LPA receptors might therefore represent interesting targets to improve the therapeutic strategies against type 2 diabetic nephropathy.
Parfois, un autre état de santé peut affecter les muscles qui mèneront à la dysfonction érectile. Dans notre génération il ya des médicaments variés pour concerner la démence, l’anxiété ou les dermatoses du cuir chevelu. Parlons de médicaments divers. Qu’en est-il de la vie sexuelle et “Impuissance masculine“? Quelle est l’examen la plus importante lequel vous avez à étudier sur “Les causes de l’impuissance masculine“? La question très importante que vous devez rechercher est ‘Les causes psychologiques de l’impuissance sexuelle‘. Généralement, cela peut inclure le diabète, une maladie rénale ou un trouble panique d’une certaine sorte. Avant de choisir le Kamagra, dites à votre docteur si vous avez des problèmes avec le sang comme le myélome multiple. Votre médecine est pour vous uniquement. Ne le donnez jamais à d’autres personnes même si leurs plaintes sont les mêmes quel les vôtres.